Ranjan Jana , Ph.D.
Senior Principal Scientist
Organic & Medicinal Chemistry

Research Focus
C-H Activation, Asymmetric Catalysis, Smart Transformation of CO2, Green Chemistry, Visible-Light-Mediated Photoredox Catalysis
Research Interest
Development of privileged medicinal scaffolds is the key step in drug discovery program. We are involved in a cutting-edge C-H activation technology for the synthesis of heterocycles and other medicinally relevant molecules. This technology is particularly important for the late-stage modification of functional molecules to impose desired properties. Furthermore, multiple C-H activation in a cascade manner will enable us to achieve molecular diversity as well as complexity from simple, readily available, inexpensive starting materials. This approach will generate library of multifunctional molecules for Alzheimer’s Disease, breast cancer research. Simultaneously, we are developing green chemical transformations from biomass and other renewable recourses to generate value added products such as fine and bulk chemicals with a particular interest in the activation and utilization of CO2 for value added products. Combining these two technologies we are developing cost effective and green chemical processes for the off-patent drugs, active pharmaceutical ingredients (APIs) and agrochemicals.
Credentials
- Principal Scientist, (2015-till date) Organic and Medicinal Chemistry Division, CSIR-IICB
- Assistant Professor, Academy of Scientific and Innovative Research (AcSIR)
- Adjunct Faculty, National Institute of Pharmaceutical Education and Research (NIPER), Kolkata
- Sr. Scientist, (2013-2015), Organic and Medicinal Chemistry Division, CSIR-IICB
- Postdoctoral Research (2010-2012) University of Utah, USA with Prof. M. S. Sigman
- Postdoctoral Research (2008-2010) University of Kansas, USA with Prof. J. A. Tunge
- Postdoctoral Research (2007-2008) Bar-Ilan University, Israel with Prof. S. Braverman
- Ph.D (2007) Organic Chemistry, Indian Association for the Cultivation of Science (IACS), Kolkata with Prof. B. C. Ranu
- M. Sc. (2002) Vidyasagar University, Organic Chemistry
- B.Sc. (2000) Midnapore College, Chemistry
Honours & Awards
- Fellow of the West Bengal Academy of Science and Technology (WAST), 2021
- Ramanujan Fellow, 2013-2019
- National Scholarship, 1995
Grants & Supports
- Extra Mural Research Grant, SERB, Department of Science & Technology, New Delhi
- Ramanujan Fellowship, SERB, Department of Science & Technology, New Delhi
- Council of Scientific and Industrial Research (CSIR), New Delhi
Patents & Publications
PUBLICATIONS
1.Nandi, S.; Mondal, S.; Jana, R.* Chemo- and Regioselective Benzylic C(sp3)–H Oxidation Bridging the Gap Between Hetero- and Homogeneous Copper Catalysis, 2022, iScience, accepted. doi.org/10.1016/j.isci.2022.104341.
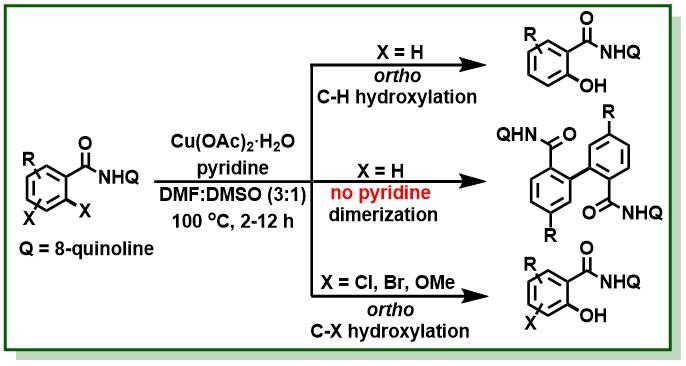
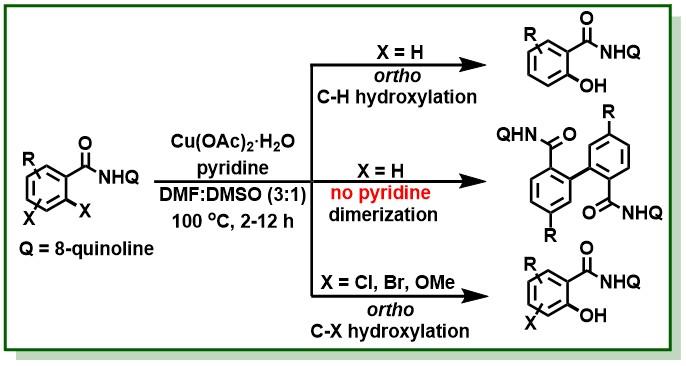
2.Begam, H. M.; Nandi, S.; Jana, R.* A Directing Group Switch in Copper-catalyzed Electrophilic C–H Amination/Migratory Annulation Cascade: Divergent Access to Benzimidazolone/Benzimidazole, Chem, Sci. 2022, 13, 5726-5733. doi.org/10.1039/D2SC01420C.
3.Das, P.; Das, S.; Jana, R.* Aryldiazonium Salts and DABSO: A Versatile Combination for Three-Component Sulfonylative Cross-Coupling Reactions, Chem. - Asian J. 2022, Ahead of Print. doi.org/10.1002/asia.202200085.
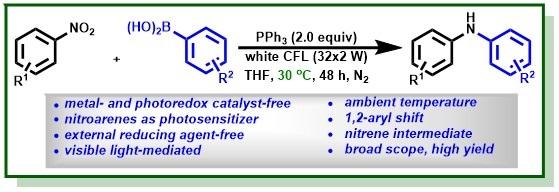
4.Manna, K.; Ganguly, T.; Baitalik, S.*; Jana, R.* Visible-Light- and PPh3-Mediated Direct C–N Coupling of Nitroarenes and Boronic Acids at Ambient Temperature, Org. Lett. 2021,23, 8634–8639.ASAP,DOI: 10.1021/acs.orglett.1c03343.
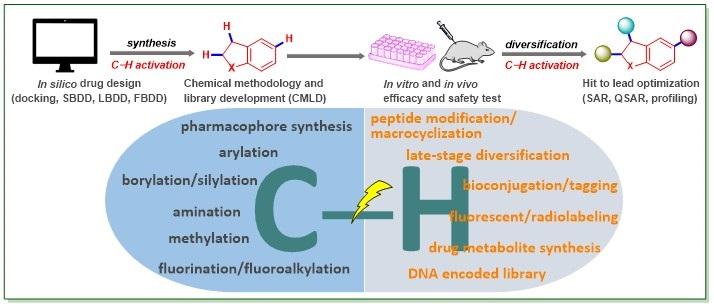
5.Jana, R.*; Begam, H. M.; Dinda, E. The emergence of the C–H functionalization strategy in medicinal chemistry and drug discovery, Chem. Commun., Feature article, 2021, 57, 10842-10866 DOI: 10.1039/D1CC04083A.
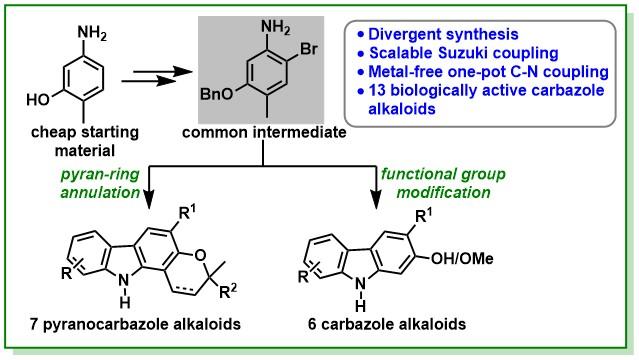
6. Polley, A.; Varalaxmi, K.; Nandi, A.; Jana, R.* Divergent Total Synthesis of (±)-Mahanine and Other Carbazole Alkaloids, Asian, J. Org. Chem. 2021, 10, 1207-1215. DOI: 10.1002/ajoc.202100176
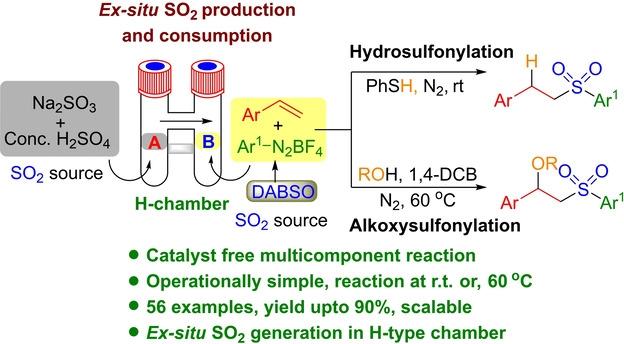
7. Das, P.; Das, S.; Varalaxmi, K.; Jana, R*. Metal-Free, Multicomponent Anti-Markovnikov Hydroarylsulfonylation and Alkoxyarylsulfonylation of Vinyl Arenes, Adv. Synth. Catal. 2021, 363, 575-584. DOI: 10.1002/adsc.202000995
8. Dinda, E.; Bhunia, S. K.; Jana, R*. Palladium-Catalyzed Cascade Reactions for Annulative π-Extension of Indoles to Carbazoles through C-H Bond Activation, Curr. Org. Chem. 2020, 24, 2612-2633. DOI : 10.2174/1385272824999200817170058
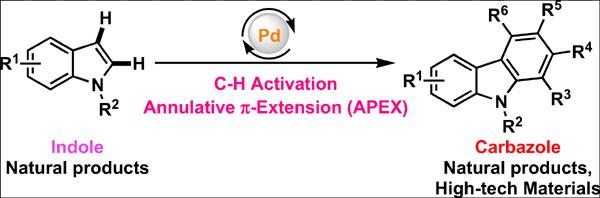
9. Manna, K.; Begam, H. M.; Samanta, K.; Jana, R. Overcoming the Deallylation Problem: Palladium(II)-Catalyzed Chemo-, Regio-, and Stereoselective Allylic Oxidation of Aryl Allyl Ether, Amine, and Amino Acids, Org. Lett. 2020, 22, 7443-7449. DOI: 10.1021/acs.orglett.0c02465
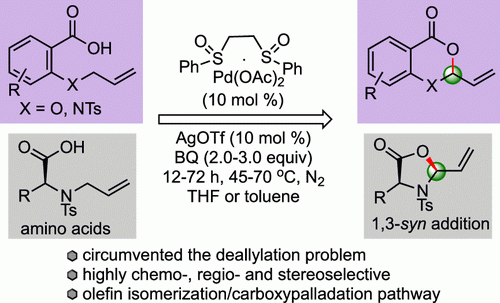
10. Das, P.; Begam, H. M.; Bhunia, S. K.; Jana, R.* Photoredox‐Catalyzed Tandem Demethylation of N,N‐Dimethyl Anilines Followed by Amidation with α‐Keto or Alkynyl Carboxylic Acids, Adv. Synth. Catal. Early view. DOI:10.1002/adsc.201900525
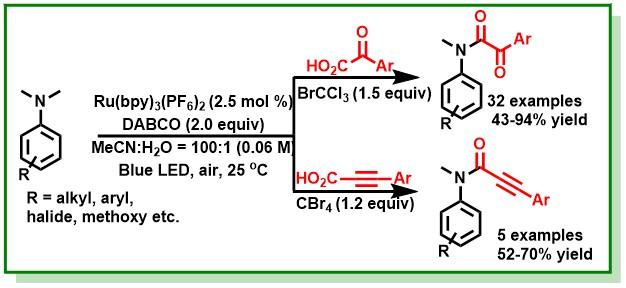
8. Bhunia, S. K.; Das, P.; Nandi, S.; Jana, R.*, Carboxylation of Aryl Triflates with CO2 Merging Palladium and Visible-Light-Photoredox Catalysts, Org. Lett. 2019, 21 (12), 4632-4637. DOI:10.1021/acs.orglett.9b01532

9. Hasina, M. B.; Choudhury, R.; Behera, A.; Jana, R.*, Copper-Catalyzed Electrophilic Ortho C(sp2)-H Amination of Aryl Amines: Dramatic Reactivity of Bicyclic System, Org. Lett. 2019, 21 (12), 4651-4656. DOI:10.1021/acs.orglett.9b01546

10. Bairy, G.; Nandi, A.; Manna, K.; Jana, R.*, Ruthenium(II)-Catalyzed Migratory C-H Allylation/Hydroamination Cascade for the Synthesis of Rutaecarpine Analogues, Synthesis, 2019, 51 (12), 2523-2531. DOI:10.1055/s-0037-1611525 (Invited special issue “Ruthenium in organic synthesis”)
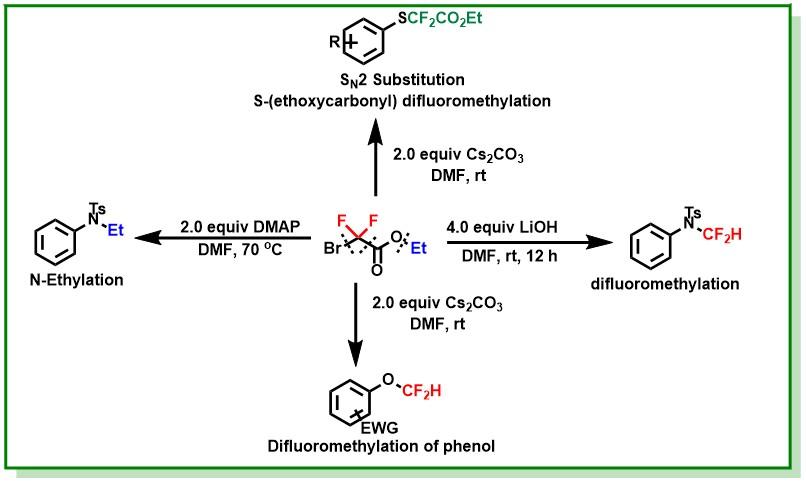
11. Bhunia, S. K.; Das, P.; Jana, R.*, Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature. Org. Biomol. Chem. 2018, 16 (47), 9243-9250. DOI:10.1039/c8ob02792g
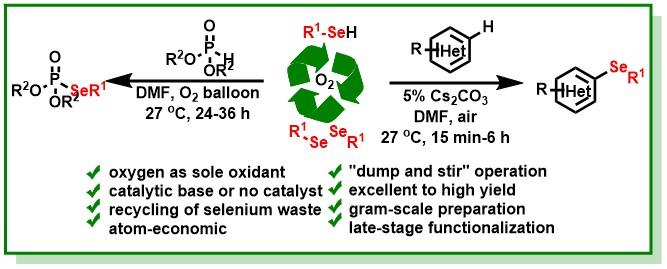
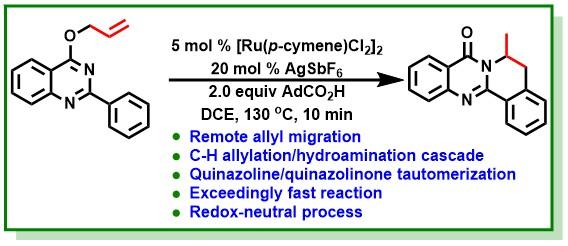
12. Bairy, G.; Das, S.; Begam, H. M.; Jana, R.*, Exceedingly Fast, Direct Access to Dihydroisoquinolino[1,2-b]quinazolinones through a Ruthenium(II)-Catalyzed Redox-Neutral C-H Allylation/Hydroamination Cascade. Org. Lett. 2018, 20 (22), 7107-7112. DOI:10.1021/acs.orglett.8b03048
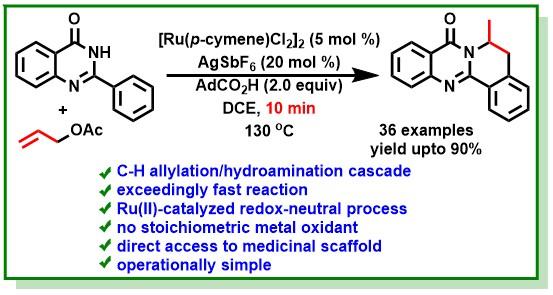
13. Polley, A.; Varalaxmi, K.; Jana, R.*, Palladium-Catalyzed Ortho C-H Arylation of Aniline Carbamates with Diazonium Salts under Mild Conditions: Expedient Synthesis of Carbazole Alkaloids. ACS Omega 2018, 3 (10), 14503-14516. DOI:10.1021/acsomega.8b02009
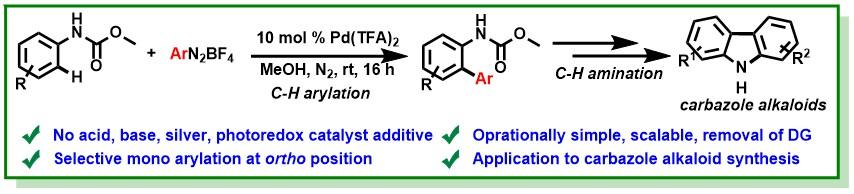
14. Polley, A.; Bairy, G.; Das, P.; Jana, R.*, Triple Mode of Alkylation with Ethyl Bromodifluoroacetate: N, or O-Difluoromethylation, N-Ethylation and S-(ethoxycarbonyl)difluoromethylation. Adv. Synth. Catal. 2018, 360 (21), 4161-4167. DOI:10.1002/adsc.201800824
15. Manna, M. K.; Bairy, G.; Jana, R.*, Sterically Controlled Ru(II)-Catalyzed Divergent Synthesis of 2-Methylindoles and Indolines through a C-H Allylation/Cyclization Cascade. J. Org. Chem. 2018, 83 (15), 8390-8400. DOI:10.1021/acs.joc.8b01034
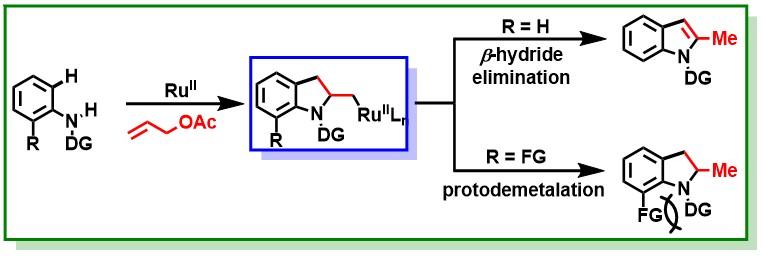
16. Hossian, A.; Manna, K.; Das, P.; Jana, R.*, CuI/AgI-Promoted Decarboxylative Alkynylation of ortho-Nitrobenzoic Acids. Chemistry Select 2018, 3 (16), 4315-4318. DOI:10.1002/slct.201800758
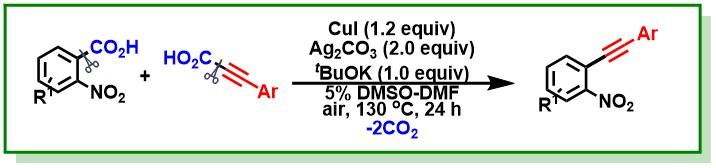
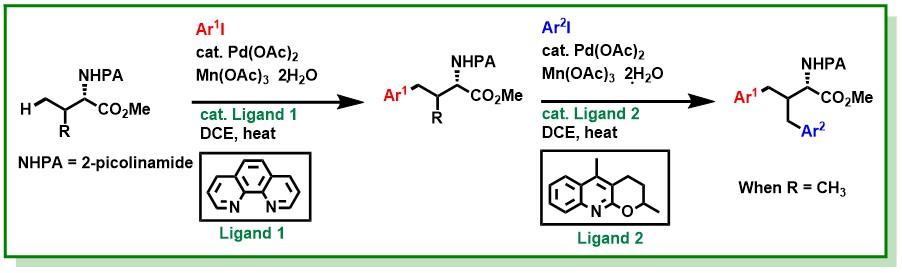
17. Das, S.; Bairy, G.; Jana, R.*, Ligand-promoted γ-C(sp3)-H arylation and unsymmetrical diarylation to access unnatural amino acid derivatives. Org. Lett. 2018, 20 (9), 2667-2671. DOI:10.1021/acs.orglett.8b00874
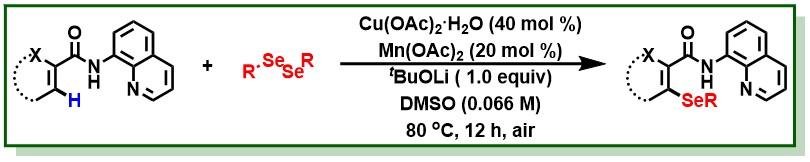
18. Singh, B. K.; Bairy, G.; Jana, R.*, A General Copper/Manganese Cocatalyzed C-H Selenation of Arenes, Heteroarenes, and Alkenes under Air, Chemistry Select, 2017, 2, 9227-9232. DOI:10.1002/slct.201701758
19. Hossian, A.; Manna, M. K.; Manna, K.; Jana, R.*, Palladium-catalyzed decarboxylative, decarbonylative and dehydrogenative C(sp2)-H acylation at room temperature, Org. Biomol. Chem. 2017, 15, 6592-6603. DOI:10.1039/C7OB01466J
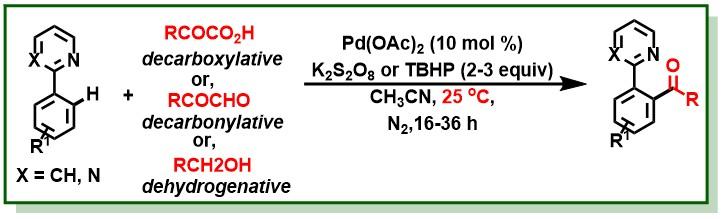
20. Manna, M. K.; Bairy, G.; Jana, R.*, Dual visible-light photoredox and palladium(II) catalysis for dehydrogenative C2-acylation of indoles at room temperature, Org. Biomol. Chem. 2017, 15, 5899-5903. DOI:10.1039/C7OB01418J
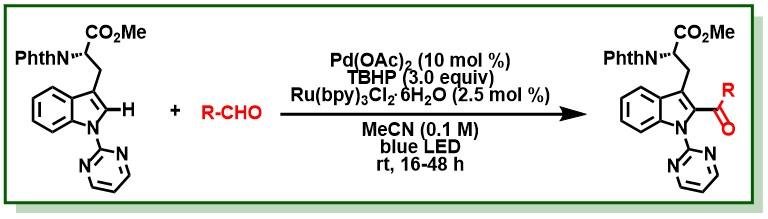
19. Manna, M. K.; Bhunia, S. K.; Jana, R.*, Ruthenium(II)-catalyzed intermolecular synthesis of 2-arylindolines through C-H activation/oxidative cyclization cascade, Chem. Commun. 2017, 53, 6906-6909. DOI:10.1039/C7CC03053C
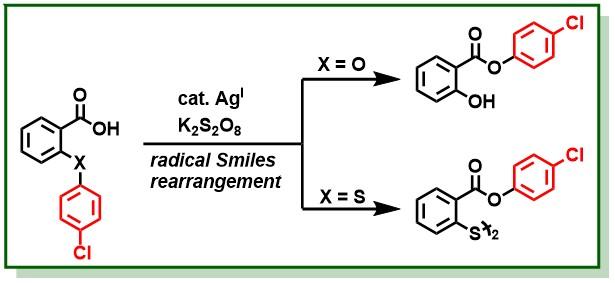
20. Hossian, A.; Jana, R.*, Carboxyl radical-assisted 1,5-aryl migration through Smiles rearrangement, Org. Biomol. Chem. 2016,14, 9768-9779. DOI:10.1039/C6OB01758D
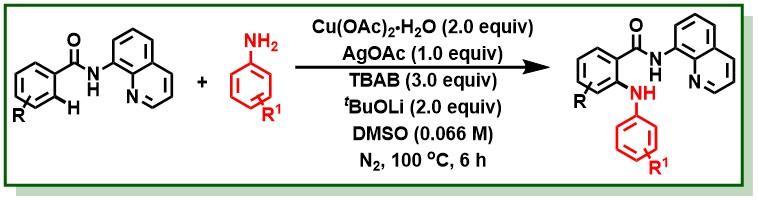
21. Singh, B. K.; Polley, A.; Jana, R.*, Copper(II)-Mediated Intermolecular C(sp2)−H Amination of Benzamides with Electron-Rich Anilines, J. Org. Chem. 2016, 81, 4295–4303. (one of the most read articles) DOI:10.1021/acs.joc.6b00659
22. Hossian, A.; Bhunia, S. K.; Jana, R.*, Substrate-Dependent Mechanistic Divergence in Decarboxylative Heck Reaction at Room Temperature, J. Org. Chem. 2016, 81, 2521-2533. DOI:10.1021/acs.joc.6b00100
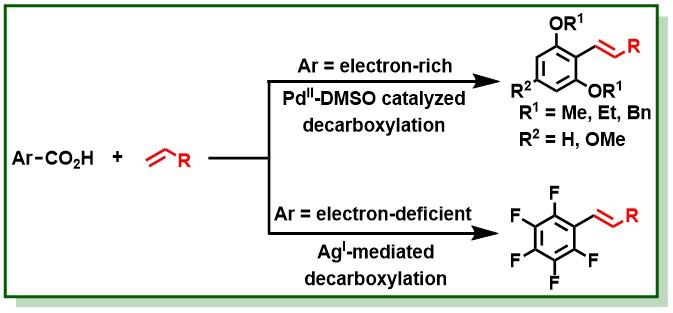
23. Singh, B. K. S.; Jana, R.*, Ligand-Enabled, Copper-Promoted Regio- and Chemoselective Hydroxylation of Arenes, Aryl Halides, and Aryl Methyl Ethers, J. Org. Chem. 2016, 81, 831–841. DOI:10.1021/acs.joc.5b02302
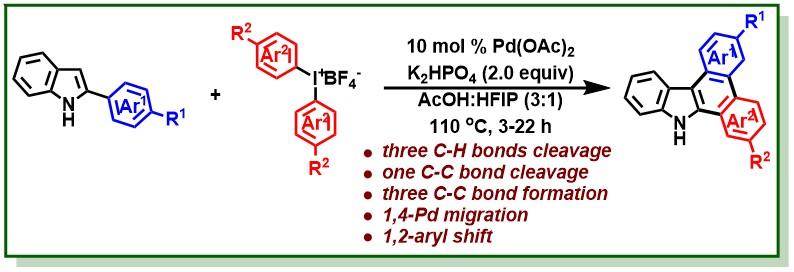
24. Bhunia, S. K.; Polley, A.; Natarajan, R.; Jana, R.*, Through-Space 1,4-Palladium Migration and 1,2-Aryl Shift: Direct Access to Dibenzo[a,c]carbazoles through a Triple C-H Functionalization Cascade- Chem. Eur. J. 2015, 21, 16786-16791. DOI:10.1002/chem.201503474
25. Chaudhary, T. Y.; Hossian, A.; Manna, M. K.; Jana, R.*, Chemo-, regio-, and stereoselective Heck–Matsuda arylation of allylic alcohols under mild conditions- Org. Biomol. Chem. 2015,13, 4841-4845. DOI:10.1039/C5OB00235D
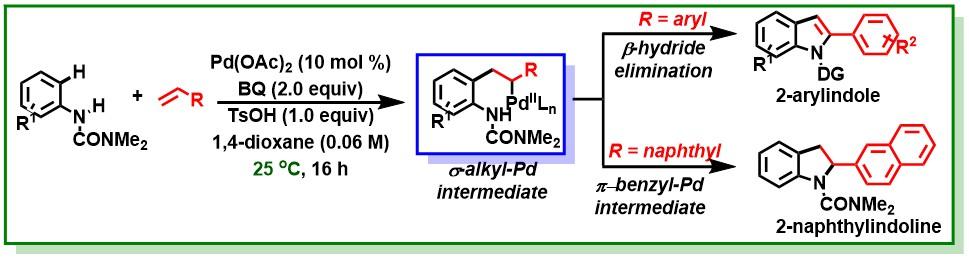
26. Manna, M. K.; Hossian, A.; Jana, R.*, Merging C−H Activation and Alkene Difunctionalization at Room Temperature: A Palladium-Catalyzed Divergent Synthesis of Indoles and Indolines- Org. Lett. 2015, 17, 672-675. (highlighted in organic chemistry portal). DOI:10.1021/ol5036968
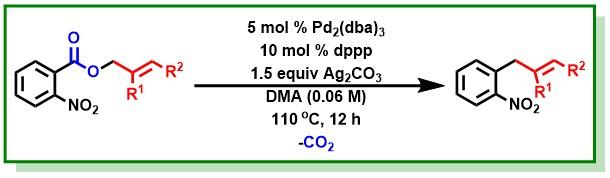
27. Hossian, A.; Singha, S.; Jana, R.*, Palladium(0)-Catalyzed Intramolecular Decarboxylative Allylation of Ortho Nitrobenzoic Esters- Org. Lett. 2014, 16, 3934-3937. DOI:10.1021/ol5017349
28. Braverman, S.*; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity- Synthesis, 2014, 46, 119-125.
29. Vaden, R. M.; Gligorich, K. M.; Jana, R.; Sigman, M. S.; Welm, B. E. The small molecule C-6 is selectively cytotoxic against breast cancer cells and its biological action is characterized by mitochondrial defects and endoplasmic reticulum stress- Breast Cancer Research, 2014, 16, 472.
30. Saini, V.; Liao. L.; Wang, Q.; Jana, R.; Sigman, M. S. Pd(0)-Catalyzed 1,1-Diarylation of Ethylene and Allylic Carbonates- Org. Lett. 2013, 15, 5008-5011.
31. Jana, R.; Pathak, T. P.; Jensen, K. H.; Sigman, M. S. Palladium(II)-Catalyzed Enantio- and Diastereoselective Synthesis of Pyrrolidine Derivatives, Org. Lett, 2012, 14, 4074-4077.
32. Jana, R.; Tunge, J. A., A Novel Recyclable Polymer Supported Rhodium(I) Catalyst for the C-C Bond Formation- J. Org. Chem. 2011, 76, 8376-8385.
33. Jana, R.; Partridge, J. J.; Tunge, J. A. Migratory Decarboxylative Coupling of Coumarins: Synthetic and Mechanistic Aspects- Angew. Chem. Int. Ed. 2011, 50, 5157-5161.
34. Liao, L.; Jana, R.; Urkalan, K. B.; Sigman, M. S. A Palladium-Catalyzed Three Component Cross-Coupling of Conjugated Dienes or Terminal Alkenes with Vinyl Triflates and Boronic Acids- J. Am. Chem. Soc. 2011, 133, 584-5787.
35. Jana, R.; Pathak, T. P.; Sigman, M. S., Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners- Chem. Rev. 2011, 111, 1417-1492.
36. Fang, J. ; Jana, R.; Tunge, J. A.; Subramaniam, B., Continuous Homogeneous Hydroformylation with Bulky Rhodium-catalyst complexes retained by nano-filtration membranes- Applied Catalysis, A: General, 2011, 393, 294-301.
37. Braverman, S.; Cherkinsky, M.; Jana, R.; Kalendar, Y.; Sprecher, M. Reaction of Selenium and Tellurium Halides with Propargyl Alcohols. The Regio and Stereoselectivity of Addition to the Triple Bond- J. Phys. Org. Chem. 2010, 23, 1114-1120.
38. Chattopadhyay, K.; Jana, R.; Day, V.; Douglas, J.; Tunge, J. A. Mechanistic Origin of the Stereodivergence in Decarboxylative allylation- Org. Lett. 2010, 12, 3042-3045.
39. Pahadi, N. K.; Paley, M.; Jana, R.; Waetzig, S. R.; Tunge, J. A. Formation of N-alkylpyrroles via Intermolecular Redox Amination- J. Am. Chem. Soc. 2009, 131, 16626-16627.
40. Jana, R.; Trivedi, R.; Tunge, J. A., Mild Decarboxylative Allylation of Coumarins- Org. Lett. 11(15), 2009, 3434-3436.
41. Duan, S.; Jana, R.; Tunge, J. A., Lewis Acid Catalyzed Diastereoselective Hydroarylation of benzylidene Malonic Esters, J. Org. Chem. 2009, 74, 4612-4614.
42. Jana, R.; Tunge, J. A., A Homogeneous, Recyclable Rhodium(I) Catalyst for the Hydroarylation of Michael Acceptors- Org. Lett. 2009, 11, 971-974.
43. Samanta, S.; Adak, L. K.; Jana, R.; Mostafa, G.; Tuononen, H. M.; Ranu, B. C.; Goswami, S., Oxidative ortho-C-N Fusion of Aniline by OsO4. Isolation, Characterization of Oxo-Amido Osmium(VI) Complexes, and their Catalytic Activities for Oxidative C-C Bond Cleavage of Unsaturated Hydrocarbons- Inorg. Chem. 2008, 47(23), 11062-11070.
44. Ranu, B. C.; Chattopadhyay, K.; Ghosh, S.; Jana, R., Ionic-liquid promoted regio- and stereoselective addition of thiols to alkynes and alkenes- J. Indian Chem. Soc. 2008, 1199-1204.
45. Ranu, B. C.; Saha, A.; Jana, R., Microwave-assisted simple and efficient ligand-free copper nanoparticle catalyzed aryl-sulfur bond formation, Adv. Synth. Catal. 2007, 349, 2690-2696.
46. Braverman, S.; Jana. R.; Cherkinsky, M.; Gottlieb, H, E.; Sprecher, M. Regio- and stereospecific synthesis of functionalized divinyl selenides- Synlett, 2007, (17), 2663-2666.
47. Ranu, B. C.; Chattopadhyay, K.; Jana, R., Chemo-, Regio- and Stereoselective Addition of Triorganoindium reagents to acetates of Baylis- Hillman Adducts: A New Strategy for the synthesis of (E)- and (Z)- trisubstituted alkenes- Tetrahedron Lett. 2007, 48(22), 3847-3850.
48. Ranu, B. C.; Jana, R.; Sowmiah, S., An Improved Procedure for the Three-Component Synthesis of Highly Substituted Pyridines Using Ionic Liquid- J. Org. Chem. 2007, 72(8). 3152- 3154.
49. Ranu, B. C.; Banerjee, S.; Jana, R., Ionic Liquid as Catalyst and Reaction medium. The Remarkable Effect of a Basic Ionic Liquid, [bmIm]OH on Michael Addition and Alkylation of Active Methylene Compounds- Tetrahedron, 2006, 63(3), 776–782.
50. Ranu, B. C.; Chattopadhyay, K.; Jana, R., Ionic liquid promoted selective debromination of α-bromoketones under microwave irradiation- Tetrahedron 2006, 63(1), 155 - 159.
51. Ranu, B. C.; Jana. R., Ionic Liquid as Catalyst and Reaction Medium – A Simple, Efficient and Green Procedure for Knoevenagel Condensation of Aliphatic and Aromatic Carbonyl Compounds Using a Task-specific Basic Ionic Liquid, Eur. J. Org. Chem. 2006, (16), 3767-3770.
52. Ranu, B. C.; Jana, R., Ionic Liquid as Catalyst and Reaction Medium: A Simple, Convenient and Green Procedure for the Synthesis of Thioethers, Thioesters and Dithianes using an Inexpensive Ionic Liquid, [pmIm]Br, Adv. Synth. Catal. 2005, 347(14), 1811-1818.
53. Ranu, B. C.; Jana, R., Catalysis by Ionic Liquid. A Green Protocol for the Stereoselective Debromination of vicinal- Dibromides by [pmIm]BF4 under Microwave Irradiation- J. Org. Chem. 2005, 70(21), 8621-8624.
54. Ranu, B. C.; Jana, R., Direct Halogenation of Alcohols and Their Derivatives with tert-Butyl Halides in the Ionic Liquid [pmIm]Br under Sonication Condition – A novel, Efficient and Green Methodology, Eur. J. Org. Chem. 2005, 4,755-758.
55. Ranu, B. C.; Jana, R.; Samanta, S., A Simple, Efficient and General Procedure for Acetalization of Carbonyl Compounds and Deprotection of Acetals under the Catalysis of Indium(III) Chloride, Adv. Synth. Catal. 2004, 346(4), 446-450.
56. Ranu, B. C.; Jana, R.; Dey, S. S., An Efficient and Green Synthesis of 2-Arylbenzothiazoles in an Ionic Liquid, [pmIm]Br under Microwave Irradiation- Chem. Lett. 2004, 33(3), 274-275.
PATENTS
1. Polymer-supported Transition Metal Catalyst Complexes and Methods of Use- Tunge, J. A.; Subramaniam, B.; Fang, J.; Jana, R. U.S. Patent, No WO 2010057099.
Products
- Developed a catalyst called JanaPhos following my name for biodiesel production from agricultural feedstocks.
