Saikat Majumder, Ph.D
Senior Scientist
Infectious Diseases and Immunology

Research Focus
Greetings from our laboratory! Immune cells are excellent candidates for studying how cellular metabolism influences functional outcome, as cells undergo adaptive metabolic adaptations throughout their development. Our research lies at the intersection of metabolism and immunity, where we expand our understanding of how changes in metabolism influence disease outcome during chronic autoimmune and infectious diseases. Our group’s goal is to pin down the factors derived from host metabolism and the environment (auto-antigen, infection, etc.) that drive disease progression and pathogenesis. We anticipate that this research will contribute to our understanding of the underlying causes of diseases that are defined by a skewed balance between the host metabolism and the immune system such as Inflammatory bowel disease (IBD), arthritis, chronic kidney diseases, etc. Our overarching goal is to investigate the potential of innovative nutritional interventions and focused metabolic modifications as therapeutic approaches for advancing the current treatment regimen.
Research Interest
Autoimmune diseases:
Apart from immune cells like T cells and macrophages, our immunity is also modulated by non-immune cells like stromal cells. Stromal cells such as fibroblastic reticular cells (FRC) provide leukocytes not only with a 3-D migratory scaffold but with important survival signals inside lymphoid organs such as lymph nodes. There is growing body of evidence (including my work), suggesting that stromal cells regulate the homeostasis and metabolism of immune cells such as T cells during chronic inflammatory conditions. We will explore the critical roles played by these cells, and attempt to manipulate them to regulate/alter their cellular functions during different human diseases.
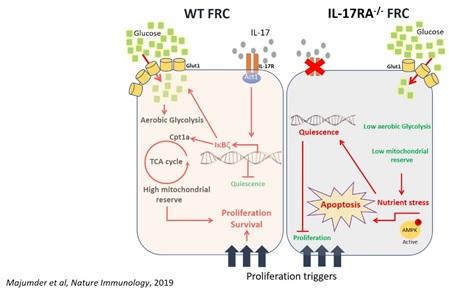
Infectious diseases:
Intracellular pathogens often hijack the host metabolism to proliferate and survive in the harsh cellular microenvironment of phagocytic cells. Determination of these altered host metabolic pathways targeted by the pathogens to survive intracellularly is critical, as they may provide newer therapeutic targets. Initially, we would like to start with the Leishmania-Macrophage infection model system, primarily because, it is an extensively studied model system to study host-pathogen interaction with multiple defined axes and immune outcomes. Down the line, some of the mechanisms uncovered will be extended into other bacterial and chronic viral infections.
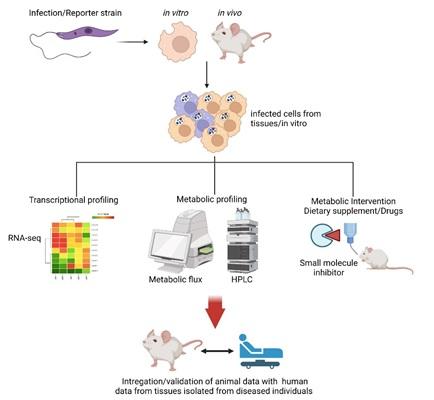
To work on these novel, cross-disciplinary, and highly collaborative projects, lab members are expected to be adaptable and open to ideas.
Interested Ph.D. students and post-docs with their own funding are encouraged to apply in accordance with the established norms of CSIR-Indian Institute of Chemical Biology.
Credentials
- Senior Scientist, Infectious Diseases & Immunology, CSIR- Indian Institute of Chemical Biology, Kolkata (2023-present)
- Senior Scientist, Bristol Myers Squibb, Boston, USA (2022-2023)
- Staff Research Scientist, University of Pittsburgh, Pittsburgh, USA (2021-2022)
- Postdoctoral Associate, University of Pittsburgh, Pittsburgh, USA (2016-2021)
- Research Associate, Bose Institute, Kolkata (2014-2016)
- PhD, Bose Institute, Kolkata (2008-2014)
Honours & Awards
- The AAI Careers in Immunology Fellowship from the American Association of Immunologists (AAI), September (2019).
- ICIS travel grant from International Cytokine & Interferon Society for attending ICIS annual meeting in Boston, USA (2018).
- Best Post-Doctoral Fellow Research Award in Department of Medicine, Annual research day 2018, University of Pittsburgh, April (2018).
- Best Postdoctoral research Award in the Department of Immunology 15th Annual Scientific Retreat in Pittsburgh, September, (2017).
- AAI travel grant from American association of Immunologists for attending AAI annual meeting in Washington DC, USA (2017).
- Awarded Marie Curie BeIPD-COFUND Postdoctoral Fellowship at Université de Liège, Belgium (2015). Declined
- EMBO fellowship for attending “EMBO Molecular medicine workshop on sphingolipids”, in Ramot, Israel, (2012).
- ‘Prof. B.B. Biswas Outstanding Student Award’ from Bose Institute for outstanding doctoral work, (2014).
- Foreign travel grant for Young Scientist from Department of Science and Technology (2013).
- Foreign travel grant for Young Scientist from Council of Scientific and Industrial Research (2012).
- Junior and Senior Research Fellowship from Council of Scientific and Industrial Research, Government of India (2008 and 2010).
- Qualified ‘Graduate Aptitude Test in Engineering’ from Ministry of Human Resource Development, Government of India (2008).
Membership
- American Association of Immunologists (AAI) (2016-present)
- International Cytokine & Interferon Society (2017-present)
Grants & Supports
- The pathogenic role of neutrophils in Type 1 diabetes. Indian Council of Medical Research (ICMR), (2025-2028).
- Interplay between IL-17 and microbiome in Ulcerative Colitis (IBD). Anusandhan National Research Foundation (ANRF), (2025-2028).
Patents & Publications
- Majumder S, Amatya N, Revu S, Jawale CV, Wu D, Rittenhouse N, Menk A, Kupul S, Du F, Raphael I, Bhattacharjee A, Siebenlist U, Hand TW, Delgoffe GM, Poholek AC, Gaffen SL, Biswas PS, McGeachy MJ. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat Immunol. May; 20(5):534-545 (2019). Commentaries featured in: a. Bordon, Y. Stromal support from IL-17. Nat Rev Immunol 19, 270–271 (2019). b. Mueller, S.N. IL-17 instructs lymphoid stromal cells. Nat Immunol 20, 524–526 (2019).
- Majumder S and McGeachy MJ. IL-17 in the pathogenesis of disease: Good intentions gone awry. Annu Rev Immunol. 26; 39:537-556 (2021).
- Wu D, Poholek CH, Majumder S, Liu Q, Revu S, Mohib K, Rothstein DM, McGeachy MJ. IL-17–dependent fibroblastic reticular cell training boosts tissue protective mucosal immunity through IL-10–producing B cells. Science Immunology.17;6(66): eaao3669 (2021).
- Poholek CH*, Raphael I*, Wu D, Revu S, Rittenhouse N, Uche UU, Majumder S, Kane LP, Poholek AC, McGeachy MJ. Noncanonical STAT3 activity sustains pathogenic Th17 proliferation and cytokine response to antigen. J Exp Med. 217(10): e20191761 (2020).
- Bechara R, Amatya N, Majumder S, Zhou C, Taylor T, Coleman B, McGeachy MJ, Gaffen SL. The RNA-binding protein IMP2 promotes IL-17 driven autoimmunity through regulation of Th17 cells generation. JCI Insight. 16: e152766 (2021).
- Taylor TC, Li Y, Li DD, Majumder S, McGeachy MJ, Biswas PS, Gingras S, Gaffen SL. Arid5a Mediates an IL-17-Dependent Pathway That Drives Autoimmunity but Not Antifungal Host Defense. J Immunol. 209(6):1138-1145 (2022).
- Du F, Garg AV, Kosar K, Majumder S, Kugler DG, Mir GH, Maggio M, Henkel M, Lacy-Hulbert A, McGeachy MJ. Inflammatory Th17 Cells Express Integrin αvβ3 for Pathogenic Function. Cell Rep. 16(5):1339-1351 (2016).
- Majumder S, Bhattacharjee A, Paul Chowdhury B, Bhattacharyya Majumdar S, Majumdar S. Antigen-Pulsed CpG-ODN-Activated Dendritic Cells Induce Host-Protective Immune Response by Regulating the T Regulatory Cell Functioning in Leishmania donavani-Infected Mice: Critical Role of CXCL10. Front Immunol. 4; 5:261 (2014).
- Majumder S, Bhattacharjee S, Paul Chowdhury B, Majumdar S. CXCL10 is critical for the generation of protective CD8 T cell response induced by antigen pulsed CpG-ODN activated dendritic cells. PLoS One. 7(11): e48727 (2012).
- Majumder S*, Dey R*, Bhattacharjee S, Rub A, Gupta G, Bhattacharyya Majumdar S, Saha B, Majumdar S. Leishmania-induced biphasic ceramide generation in macrophages is crucial for uptake and survival of the parasite. J Infect Dis. 15; 205(10):1607-16 (2012).
* Equal contributors
Complete list of published work:
Google scholar link: https://scholar.google.com/citations?user=e4eTDOcAAAAJ&hl=en&oi=ao
