Sanjay Dutta , Ph.D.
Senior Principal Scientist
Organic & Medicinal Chemistry

Research Focus
The focus of our laboratory is in interdisciplinary science in translation research involving chemical biology which will have an impact on the health and diseases.
Research Interest
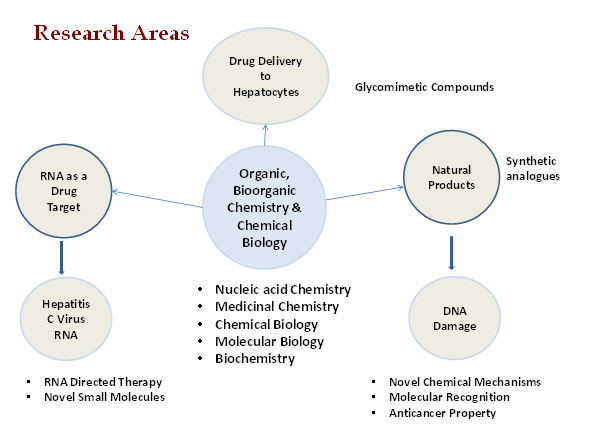
Currently four different research areas are there in our laboratory
- Antivirals against (Hepatitis C Virus)
- DNA damage and Cancer
- Targeting the mTOR pathway for (Lipogenesis and Lipid accumulation)
- Development of Novel Antibacterials
1. Objective: My laboratory at CSIR-IICB at Kolkata is involved in the development of novel antivirals targeting Hepatitis C Virus RNA and the liver specific delivery of these antivirals.
Hepatitis C Virus (HCV) infection is one of the major liver diseases and is a global health concern. HCV infection affects almost 180 million people worldwide which represent almost 3% of the world population. HCV mainly effects liver, pathophysiological symptoms associated with this disease is liver cirrhosis, liver failure and hepatocellular carcinoma. Absence of vaccine and inability to detect virus load in acute condition is major reason for liver transplantation. Recent studies have shown that the Internal Ribosome Entry Site (IRES) RNA of 5’-untranslated region (UTR) is a target for antiviral development due to the highly conserved region of HCV with unique structural features. Till date absolute therapy of Hepatitis C virus is problematic. There is an urgent need for the development of antivirals targeting HCV infection. My laboratory at IICB-Kolkata is engaged in the discovery and development of novel antivirals targeting Hepatitis C virus RNA.
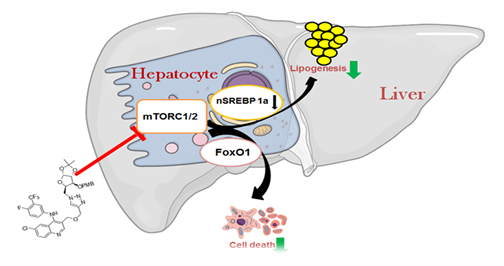
2. Objective: Reduction of lipogenesis and lipid accumulation by glycomimetic-quinoline conjugates via mTOR pathway in Hepatocytes
Principal Investigator: Dr. Sanjay Dutta, Senior Scientist, CSIR-Indian Institute of Chemical Biology, Kolkata. Co-Investigator: Dr. Partha Chakrabarti, Principal Scientist, CSIR-Indian Institute of Chemical Biology, Kolkata
(ChemBioChem 2018, 19, 1720–1726)
Abstract: Nonalcoholic fatty liver disease (NAFLD), which is characterized by excess accumulation of triglyceride in hepatocytes, is the major cause of chronic liver disease worldwide and no approved drug is available. The mechanistic target of rapamycin (mTOR) complexes has been implicated in promoting lipogenesis and fat accumulation in the liver, and thus, serve as attractive drug targets. The generation of non- or low cytotoxic mTOR inhibitors is required because existing cytotoxic mTOR inhibitors are not useful for NAFLD therapy. New compounds based on the privileged adenosine triphosphate (ATP) site binder quinoline scaffold conjugated to glucose and galactosamine derivatives, which have significantly low cytotoxicity, but strong mTORC1 inhibitory activity at low micromolar concentrations, have been synthesized. These compounds also effectively inhibit the rate of lipogenesis and lipid accumulation in cultured hepatocytes. This is the first report of glycomimetic quinoline derivatives that reduce lipid load in hepatocytes. (ChemBioChem 2018, 19,1720–1726).
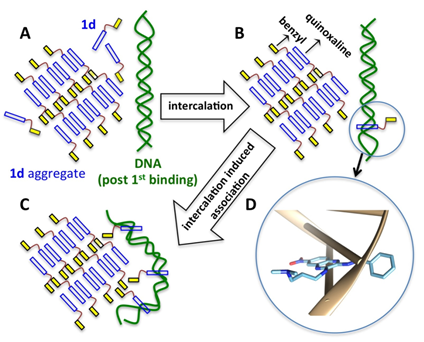
3. Quinoxaline based small molecules targeting DNA
(Angew. Chem. Int. Ed. 2016, 55, 7733-7736, doi:10.1002/anie.201511881.)
Abstract: Small molecules that intercalate DNA have tremendous therapeutic potential. Typically, DNA intercalators do not alter the overall DNA double-helical structure, except locally at the intercalation sites. In a previous report, we showed that a quinoxaline-based intercalator with a mandatory benzyl substitution (1d) induced an unusually large circular dichroism signal upon DNA binding, suggesting the formation of intercalated DNA superstructures. However, no detailed structural studies have been reported. Using atomic force microscopy, we have probed the nature of the superstructure and report the formation of a plectonemically oversupercoiled structure of pBR322 plasmid DNA by 1d, where close association of distant DNA double-helical stretches is the predominant motif. Without the benzyl moiety (1a), no such DNA superstructure was observed. Similar superstructures were also observed with doxorubicin (dox), a therapeutically important DNA intercalator, suggesting that the superstructure is common to some intercalators. The superstructure formation, for both intercalators, was observed to be GC-specific. Interestingly, at higher concentrations (1d and dox), the DNA superstructure led to DNA condensation, a phenomenon typically associated with polyamines but not intercalators. The superstructure may have important biological relevance in connection to a recent study in which dox was shown to evict histone at micromolar concentrations. (Biochemistry 2018, 57, 5557−5563).
We are developing DNA damaging novel small molecules which target different cancer cells. We are working on the elucidation of DNA damage pathways triggered by the small molecules.
Credentials
- Research Associate, (2011-2012), The Scripps Research Institute, La Jolla, California,USA (Prof M. G Finn)
- Post-doctoral Research Scholar (2007-2010), University of California-San Diego, California, USA (Prof. Thomas Hermann)
- Ph.D (2007) Organic Chemistry/Bio-organic Chemistry, University of Missouri, Columbia MO, USA (Prof. Kent S. Gates)
Patents & Publications
PUBLICATIONS
- Rimita Saha, Ritesh Pal, Bhaskar Ganguly, Bhim Majhi and Sanjay Dutta*. “Mono-quinoxaline-induced DNA structural alteration leads to ZBP1/RIP3/MLKL-driven necroptosis in cancer cells”. Eur J Med Chem. 2024, Apr 15; 270:116377. doi: 10.1016/j.ejmech.2024.116377. IF 6.0
- Shalmali Basu, Debashree Das, Zarina Ansari, Nabakumar Rana, Bhim Majhi, Dipendu Patra, Ajay Kanungo, David Morgan, Sanjay Dutta, Kamalika Sen* “A multispectroscopic approach for ultra-trace sensing of prostate specific antigen (PSA) by iron nanocomposite fabricated on graphene nanoplatelet.” Spectrochim Acta A Mol Biomol Spectrosc. 2023 Nov 15; 301:122955. doi: 10.1016/j.saa.2023.122955. Epub 2023 Jun 5. IF 4.831.
- Bhim Majhi,# Sudakshina Ganguly,# Subhadeep Palit,# Aymen Parwez, Rimita Saha, Gautam Basu,* and Sanjay Dutta* “Sequence-specific dual DNA binding modes and cytotoxicities of N-6 functionalized norcryptotackieine alkaloids” Journal of Natural Products, 2023, 86, 7, 1667–1676. doi: 10.1021/acs.jnatprod.2c01045 (#These authors contributed equally.) IF 4.803
- Khondakar Sayef Ahammed,# Sangita Pachal,# Papiya Majumdar* and Sanjay Dutta* “DNA morphology: Global alteration of DNA topological states induced by chemotherapeutic agents and its implication in cancer” ChemBioChem, 2023, e202200715, 1-13 doi.10.1002/cbic.202200715 (#These authors contributed equally.) IF 3.468
- Abhi Das*, Jeet Chakraborty, Soching Luikham, Sayanika Banerjee, Jhimli Bhattacharya and Sanjay Dutta* “Targeting aloe active compounds to c-KIT promoter G-quadruplex and comparative study of their anti-proliferative property”, J Biomol Struct Dyn. 2022 Nov 15:1-9. doi: 10.1080/07391102.2022. IF 4.152
- Bhim Majhi,# Aymen Parwez,# Subhadeep Palit, and Sanjay Dutta* “One-Pot Cascade Annulation-Triggered Synthesis of N‑6-Substituted Norcryptotackieine Alkaloids and Evaluation of Their Antileishmanial Activities” J. Org. Chem. 2022, 87, 14695−14705 (#These authors contributed equally.) IF 4.198
- Chandra Sova Mandi, Tridib Mahata, Dipendu Patra, Jeet Chakraborty, Achyut Bora, Ritesh Pal and Sanjay Dutta* “Cleavage of Abasic sites in DNA by an aminoquinoxaline compound: Augmented cytotoxicity and DNA damage in combination with an anticancer drug Chlorambucil in human colorectal carcinoma cells” ACS Omega 2022, 7, 8, 6488-6501 DOI 10.1021/acsomega.1c04962. IF 4.132
- Ritesh Pal,# Jeet Chakraborty,# Titas Kumar Mukhopadhyay,# Ajay Kanungo, Rimita Saha, Amit Chakraborty, Dipendu Patra, Ayan Datta* and Sanjay Dutta* “Substituent effect of benzyl moiety in nitroquinoxaline small molecules upon DNA binding: Cumulative destacking of DNA nucleobases leading to histone eviction” European Journal of Medicinal Chemistry Volume 229, 5 February 2022, 113995, https://doi.org/10.1016/j.ejmech.2021.113995. IF 7.088 (#These authors contributed equally.)
- Subhadeep Palit,# Sayanika Banerjee,# Tridib Mahata,# Sougata Niyogi, Tanusree Das, Chandra Sova Mandi, Partha Chakrabarti, Sanjay Dutta* “Interaction of a triantennary quinoline glycoconjugate with Asialoglycoprotein receptor” ChemMedChem 2021, 16, 2211–2216 https://doi.org/10.1002/cmdc.202100158. IF 3.466, (#These authors contributed equally.)
- Abhi Das* and Sanjay Dutta* “Binding Studies of Aloe-Active Compounds with G‑Quadruplex Sequences” ACS Omega, 2021, 6, 18344−18351, https://doi.org/10.1021/acsomega.1c02207. IF 3.512
- Dipendu Patra, # Sayanika Banerjee,# Chandra Sova Mandi, K. S. Haseena, Gautam Basu, and Sanjay Dutta* “A pyrimido-quinoxaline fused heterocycle lights up transfer RNA upon binding at the Mg2+ binding site” ChemBioChem 2021, 22, 359 –363, DOI:10.1002/cbic.202000584. (# These authors contributed equally.) IF 3.164
- Jeet Chakravarty, Ajay Kanungo, Tridib Mahata, Krishna Kumar, Geetika Sharma, Ritesh Pal, Khondakar Sayef Ahammed, Dipendu Patra, Bhim Majhi, Saikat Chakrabarti, Saumitra Das and Sanjay Dutta* “Quinoxaline derivatives disrupt the base stacking of Hepatitis C Virus Internal Ribosome Entry Site RNA: reduce translation and replication” Chem. Commun., 2019, 55, 14027, DOI: 10.1039/c9cc06531h. IF ~ 6.22
- Khondakar Sayef Ahammed, Ritesh Pal, Jeet Chakraborty, Ajay Kanungo, Polnati Sravani Purnima, and Sanjay Dutta* “DNA Structural Alteration Leading to Antibacterial Property of 6-Nitroquinoxaline Derivatives” J. Med. Chem., 2019, 62, 17, 7840-7856, DOI: 10.1021/acs.jmedchem.9b00599. IF ~ 7.446
- Abhi Das*, Gopinatha Suresh Kumar and Sanjay Dutta* “Interaction of aloe active compounds with calf thymus DNA” J Mol Recognit. 2019, e2786, 1-9, doi/10.1002/jmr.2786. IF ~ 2.137
- Tridib Mahata, Jeet Chakraborty, Ajay Kanungo, Dipendu Patra, Gautam Basu* and Sanjay Dutta* “Intercalator induced DNA superstructure formation: doxorubicin and a synthetic quinoxaline derivative” Biochemistry 2018, 57, 5557−5563. IF ~3.16.
- Subhadeep Palit,# Sanghamitra Mukherjee,# Sougata Niyogi,# Anindyajit Banerjee, Dipendu Patra, Amit Chakraborty, Saikat Chakrabarti, Partha Chakrabarti* and Sanjay Dutta* “Quinoline-glycomimetic conjugates reducing lipogenesis and lipid accumulation in hepatocytes” ChemBioChem, 2018, DOI: 10.1002/cbic.201800271.( #These authors contributed equally.) IF 3.164
- Carlos A. Sanhueza, Michael M. Baksh, Benjamin Thuma, Marc D. Roy, Sanjay Dutta, Cathy Preville, Boris A. Chrunyk, Kevin Beaumont, Robert Dullea, Mark Ammirati, Shenping Liu, David Gebhard, James E. Finley, Christopher T. Salatto, Amanda King-Ahmad, Ingrid Stock, Karen Atkinson, Benjamin Reidich, Wen Lin, Rajesh Kumar, Meihua Tu, Elnaz Menhaji-Klotz, David A. Price, Spiros Liras,M. G. Finn,*and Vincent Mascitti* Efficient Liver Targeting by Polyvalent Display of a Compact Ligand for the Asialoglycoprotein Receptor J. Am. Chem. Soc. 2017, 139, 3528-3536. IF ~ 15.419
- Tridib Mahata, Ajay Kanungo, Sudakshina Ganguly, Eswar Kalyan Modugula, Susobhan Choudhury, Samir Kumar Pal, Gautam Basu,* and Sanjay Dutta* The Benzyl Moiety in a Quinoxaline-Based Scaffold Acts as a DNA Intercalation Switch. (Angew. Chem. Int. Ed. 2016, 55, 7733-7736, doi:10.1002/anie.201511881.) IF ~ 15.3.
- Ajay Kanungo,‡ Dipendu Patra,‡ Sanghamitra Mukherjee, Tridib Mahata, Prakas R. Maulika and Sanjay Dutta* Synthesis of a Visibly Emissive 9-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoxalin-5-amine Scaffold with Large Stokes Shift and Live Cell Imaging, RSC Adv., 2015, 5, 70958-70967. DOI: 10.1039/C5RA12960E. (‡These authors contributed equally.) IF ~ 3.361
- Rynearson KD, Dutta S, Tran K, Dibrov SM & Hermann T. Synthesis of oxazole analogs of streptolidine lactam (2013) Eur. J. Org Chem., 7337-7342. IF ~ 3.021
- On the formation and properties of interstrand DNA-DNA cross-links forged by reaction of an abasic site with the opposing guanine residue of 5'-CAp sequences in duplex DNA. Johnson K. M, Price N. E, Wang J, Fekry M. I, Dutta S, Seiner D. R, Wang Y, Gates K. S J. Am. Chem. Soc., 2013, 135, 1015-25. IF ~ 15.419
- Glycomimetic Ligands for the Human Asialoglycoprotein Receptor. Mamidyala, S. K§.; Dutta, S§.; Chrunyk, B. A.; Préville, C.; Wang, H.; Withka, J. M.; McColl, A.; Subashi, T. A.; Hawrylik, S. J.; Griffor, M. C.; Kim, S.; Pfefferkorn, J. A.; Price, D. A.; Menhaji-Klotz, E.; Mascitti, V. and Finn. M.G. J. Am. Chem. Soc., 2012, 134, 1978–1981.(§These authors contributed equally.) IF ~ 15.419
- Synthesis and crystal structure of a novel heterocycle, 2-oxa-4,7-diazabicyclo[3.3.1]non-3-ene. Dutta, S.; Dibrov, SM.; Ho, BT.; Higginson, C. J.; & Hermann T. J. Chem.Cryst. 2012, 42, 119-129.
- Noncovalent DNA Binding Drives DNA Alkylation by Leinamycin: Evidence That the Z,E-5-(Thiazol-4-yl)-penta-2,4-dienone Moiety of the Natural Product Serves as an Atypical DNA Intercalator. Fekry, M.; Szekely, J.; Dutta, S.; Breydo, L.; Zang, H.; Gates, K. S. J. Am. Chem. Soc. 2011, 132. IF ~ 15.419
- A crystallographic study of a highly substituted imidazolinone, (3S,4S,5R)-3-(((S)-4-((1H-indol-3-yl)methyl)-5-oxo-4,5-dihydro-1H-imidazol-2-yl)amino)-4-((tert-butyldimethylsilyl)oxy)-5-hydroxypiperidin-2-one Dutta, S, Dibrov SM, Higginson CJ & Hermann T. J. Chem.Cryst. 2011, 41, 1573-1578.
- 1, 3-Diazepanes of Natural Product-Like Complexity from Cyanamide-Induced Rearrangement of Epoxy-δ-lactams. Dutta, S.; Higginson, C. J.; Bao, T. Ho.; Rynearson, K. D.; Dibrov, S. M. And Hermann, T.; Organic Letters, 2010, 12, 360-363. IF ~ 6.005
- 1-Cyclohexyl-2-(3-furyl)-1H-benzimidazole-5-carboxylic acid. Dibrov, S.; Dutta, S. and Hermann, T. Acta Cryst. (2009). E65, o2136.
- Conformational inhibition of the HCV IRES RNA. Parsons, J.; Castaldi, M. P.; Dutta, S.; Dibrov, S. M.; Wyles, D. L. and Hermann, T. Nature. Chem. Biol, 2009, 5, 823-825. (Reported in News and Views by D. W Begley & G Varani in the same Journal). IF ~ 13.942
- Solano, B.; Junnotula, V.; Martin, Adoracion.; Villar, R.; Burguette, A.; Vicente, E.; Silanes, S. P.; Aldana, I.; Mongoe, A.; Dutta, S.; Sarkar, U.; Gates, K. S. "Synthesis and Biological Evaluation of New 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide Derivatives and Their Reduced Analogue". J. Med. Chem., 2007, 50 (22), pp 5485–5492. IF ~ 7.446
- Dutta, S.; Chowdhury, G.; and Gates, K. S "Interstrand crosslinks generated by abasic sites in duplex DNA". . J. Am. Chem. Soc. 2007, 129, 1852-1853. IF ~ 15.419
- DNA Damage by Fasicularin. Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; and Gates, K. S. J. Am. Chem. Soc. 2005, 127, 15004-15005. IF ~ 15.419
- Gates, K. S.; Nooner, T and Dutta, S "Biologically Relevant Chemical Reactions of N7-Alkylguanine Residues in DNA". . Chem. Res. Toxicol, 2004, 17, 839-856. IF ~ 3.739
- Nooner, T.; Dutta, S and Gates, K. S."Chemical Properties of the Leinamycin-Guanine Adduct in DNA". Chem. Res. Toxicol, 2004, 17, 942-949. IF ~ 3.739
